The most common metals to consider when discussing recycling are aluminum and steel. Some other metals–like gold, silver, brass, and copper–are so valuable that they are rarely thrown away. They do not create a waste disposal problem.
Aluminum and steel do. Americans use 100 million steel cans and 200 million aluminum beverage cans every day (300,000,000 metal cans). What should we do with this metal waste? Should we burn it in waste-to-energy plant? Should we landfill it? Or should we recycle it?
After source reduction (using less aluminum to make a can, for example), recycling is the most efficient way to reduce aluminum and steel waste.
Unlike paper and plastics, burning metal trash in waste-to-energy plants creates no energy. Instead, aluminum melts and steel just gets very hot. Magnets can be used to collect steel scrap at waste-to-energy plants, though, and then the scrap can be shipped to steel plants for recycling.
Landfilling is usually not a good alternative either. Aluminum, in particular, is so valuable as a scrap material that it simply does not make sense to bury it.
this saves 155% of energy and 95% of air pollution
Scrap metal includes ferrous metals (iron and steel) and nonferrous materials (aluminum, copper, tin, brass). Many of our home appliances are made of metals. This includes our washers & dryers, refrigerators, ovens & stoves and water heaters. Waste from unwanted appliances can be categorized in two main types: refrigerants (Freon) and non-refrigerants.
The Process
The recycling process for metal is similar to those of other materials. It is best described in four stages:
- Collection
- Processing
- Shredding
- Selling
After collection and proper sorting, the scrap is compacted. It is then sold to minimills, which process them to steel. According to RecycleMetal.org, “processing scrap metal to steel instead of virgin ore require about 74 percent less energy.”
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of metal readily oxidizes back into metal oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2800 °F to produce molten metal.
- Dross is removed and the dissolved hydrogen is degassed. (Molten metal readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity metal, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 metal alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten metal poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
Tips for Recycling
- Have the delivery company for your new appliance, take the old with them. These companies can either recycle the unit or properly dispose of it
- Have a professional disassemble your appliance and take the appropriate materials to recyclers in your area
- If the appliance is still working, sell it on online, donate it to ta charity or give it to a friend
recycling one ton of metal could save 5/4 tons of iron ore, 1,000 pounds of coal, 278 pounds of limestone, a 465/2 cubic meter lake (2/5 of water), enough energy to light a 60 watt light bulb for 1,300/219 years, a CFL for 49,826,951/292,000 years, power over 493/2,000 cars for a year, 3,214,642 KWh of energy (37/50 of energy), a 489/80 cubic meter container of oil, 248,500,000 Btu's of energy (37/50 of energy), 14 cubic yards of landfill space, the equivalent of a 999/160 cubic meter tank of gasoline, 329/200 tons of red mud, 4,383/1,000 tons of bauxite, 1,020 pounds of petroleum coke, 966 pounds of soda ash, 327 Pounds of pitch, 29/20 tons of co2, 81 pounds of air pollution (reduced by 43/50), 789 pounds of solid waste, Reduce water pollution by 19/25, enough oil to run the average car for 24,450 miles or circle the globe almost 1,825,600 times, a 48,900,000 cubic meter lake from being polluted, and keep 800 pounds of airborne mercury, 3,600,000 tons of cyanide out of landfills, 806/73 metric tons of coal, 35,464/1,095 metric tons of co2, 6,520 acres of soil from being polluted, 80 pounds of fly ash, 403,000/5,037 tons of steam, 9,949,407/9,232 tons of biomass, 100 pounds of sulfur dioxide, 750 pounds of coke, 20,375/13 metric tons of global warming, 50 pounds of sulfur, 6,595,153/2,159 tons of acid rain, 333/2 kilograms of benzene, 12,425/969 tons of ammonia, 497/38 tons of methanol, 333/20 tons of climate change, almost 4,401/280 tons of hydrocarbons, and enough energy to power a 100-watt light bulb for 2,480/73 years, a TV for 300/73 years or a computer for 650/219 years, $396,548.04, gain 6,993/400 tons of oxygen
recycling one metric ton of metal could save up to 8 metric tons of bauxite, over 777 pounds of co2 a year, 400 metric tons of toxic lead, 4 metric tons of chemical products, 14,000 kwh of energy, over 11 metric tons of greenhouse gases, 12,950/229 gallons of oil, enough energy to power a 21/2 bedroom house for an entire year, and over 13,209/200,000 cars for a year, a CFL for 39,613/3,796 Years, one car to travel 194,250/229 miles, 777/2 power strips, save 11/10 metric tons of iron ore, 630 kilograms of coal, 55 kilograms of limestone, keep 4,599/201,500 tons of mercury out of landfills a year, 7,392/5 square meters of natural habitat potential, 105/23 tons of steam, 51,800/229 acres of soil from being polluted, enough energy to power a 100-watt light bulb for 126/65 years, a tv for 21/130 years, 6,201/61,180,745 metric tons of fossil fuels, 14/39 metric tons of hydrogen, 1,043/400 tons of carbon, 2,772/625 metric tons of life, 1,309/25,000 metric tons of solid particles
Recycling Aluminum
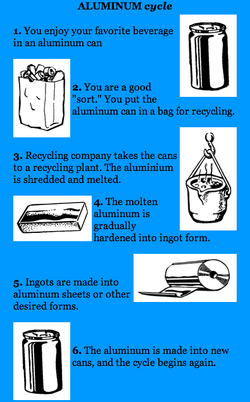
ALUMINUM cycle
- You enjoy your favorite beverage in an aluminum can
- You are a good "sort." You put the aluminum can in a bag for recycling.
- Recycling company takes the cans to a recycling plant. The aluminum is shredded and melted.
- The molten aluminum is gradually hardened into ingot form.
- Ingots are made into aluminum sheets or other desired forms.
- The aluminum is made into new cans, and the cycle begins again.
Like most metals, other than gold, aluminum is obtained from an ore. An ore is a mineral that is mined for a valuable material contained within it. Bauxite, a reddish clay-like ore, is rich in aluminum compounds.
The tricky thing about aluminum—unlike copper, iron, and other common metals—is that it only exists in combination with other elements, usually oxygen. Combined with oxygen, aluminum forms an extremely hard material known as alumina. To free the aluminum, the alumina must be stripped or reduced of its oxygen. This process is done at a reduction plant, or smelter. The alumina is put into large pots at the reduction plant. First, it is dissolved in a molten (or liquid) salt (2800 F).
Then, a powerful electric current is run through the liquid to separate the aluminum from the oxygen. The molten aluminum sinks to the bottom of the pots. The reduction process requires a tremendous amount of electrical energy.
That is why recycling aluminum makes sense. It saves energy—a lot of energy. Today, aluminum can recycling saves about 11.5 billion kilowatt-hours (kWh)—enough electricity to light a city the size of Pittsburgh for six years, $1,380,000,000.00.
As you probably know, energy is expensive! Just take a look at your electric bill, or note the price of a gallon of gasoline the next time you see a gas station. Making a pound of aluminum from bauxite ore (a pound is about how much 34 aluminum beverage cans weigh) takes 7.5 kilowatt-hours of energy, and costs 90¢.
Making aluminum from recycled aluminum scrap, on the other hand, takes only four percent of the energy—just one-third kWh. Recycling four aluminum cans saves as much energy as the energy in one cup of gasoline.
That is also why used aluminum items have a high scrap value. Aluminum manufacturers save energy and money by using recycled aluminum, so they will pay you for your old cans—about a penny for every can. One could sell scrap aluminum, or any metal for that fact, to a scrap yard aka a salvage yard. [1]
energy and air pollution is saved by 95%
recycling ton of aluminum could save the equivalent of a 999/160 cubic meter tank of gasoline, 329/200 tons of red mud, 143/100 tons of dust each year, 1,554 gallons of oil, 10 cubic yards of landfill space, 14,000 kWh of energy, 237,600,000 Btu’s of energy, 891/2 pounds of ozone each year, 4,383/1,000 tons of bauxite, 1,020 pounds of petroleum coke, 966 pounds of soda ash, 327 pounds of pitch, 238 pounds of limestone, 29/20 tons of co2, 81 pounds of air pollution, 789 pounds of solid waste, a 42 cubic meter lake, enough energy to power over 493/2,000 cars for a year, a CFL for 217/292 years, 71,280/19 homes for a year, enough oil to run the average car for 23,310 miles or circle the globe almost 1,740,480 times, 2,331/4 tons of green house gases, a 46,620,000 cubic meter lake, and 6,216 acres of soil from being polluted, 400 metric tons of toxic lead, 14/39 metric tons of hydrogen, 9,387/2,000 tons of biomass, 1,043/400 tons of carbon, a 89,100/9,169 cubic meter container on propane, 97,047/4,000,000 tons of methane, 140/39 metric tons of zinc, over 9,387/183,200 tons of smog, 10,323/10,000,000 tons of VOCs, 66,825/64 cubic meters of rain, 2,673/16,000 tons of particulates each year, 83,853/25 decibels of sound intensity, a 783/1,360 cubic meter container of biodiesel, 5,481/68,000 tons of glycerol, $3,105.60, gain almost 11,583/800 tons of oxygen per year
recycling one metric ton of aluminum could save up to 8 metric tons of bauxite, over 777 pounds of co2 a year, 4 metric tons of chemical products, 14,000 kwh of energy, over 10 metric tons of greenhouse gases, 12,950/229 gallons of oil, 51,800/229 acres of soil from being polluted, enough energy to power a 21/2 bedroom house for an entire year, and over 13,209/200,000 cars for a year, a CFL for 217/292 Years, one car to travel 194,250/229 miles, 777/2 power strips.
Steel Recycling
Steel is the most recycled material in the United States. Steel dominates the recycling mix because every year the steel industry recycles huge amounts of steel scrap from cars, appliances, and torn-down buildings and bridges. Today, all steel products are made with some recycled steel.
SAVING energy by recycling STEEL
- The average family in the United States uses 90 pounds of steel cans a year.
- Recycling that steel would save:
In 1998, the amount of steel that was recycled decreased for the first time in many years. Foreign countries were selling their steel so cheaply that the recycling industry suffered a decline. Today, it is increasing again.
You can do your part at home by recycling steel cans. A steel can is the can your soup comes in, or your dog’s food, or your mom’s coffee, or the whip cream you squirt on top of an ice-cream sundae. In fact, most food containers are made of steel.
You have probably heard many people call a steel can a tin can. Steel cans are often called tin cans because they are usually coated with a thin layer of tin. Tin protects the food that is cooked in the can.
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of Steel readily oxidizes back into Steel oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2,800 °F to produce molten steel.
- Dross is removed and the dissolved hydrogen is degassed. (Molten steel readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity steel, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 steel alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten steel poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
energy is saved by 60%
recycling one ton of steel, could save 5/4 tons of iron ore, 1,000 pounds of coal, 40 pounds of limestone, a 465/2 cubic meter lake (2/5 of water), enough energy to light a 60 watt light bulb for 1,300/219 years, a CFL for 9,951/292,000 years, 642 KWh of energy (37/50 Of energy), 76 Gallons of oil, 57/2 tons of green house gases, 10,900,000 Btu's of energy (37/50 of energy), 4 cubic yards of landfill space, reduce air pollution by 43/50, water pollution by 19/25, enough oil to run the average car for 1,140 miles or circle the globe almost 85,120 times, a 2,280,000 cubic meter lake from being polluted, 13,053/25,000 tons of co2, 642/35 metric tons of toxic lead, 304 acres of soil from being polluted, 80 pounds of fly ash, 860,922/115 cubic feet of natural gas, 214/13 kilograms of hydrogen, 430,461/2,000,000 tons of biomass, 47,829/400,000 tons of carbon, a 8,175/18,338 cubic meter container of propane, 107/650 metric tons of zinc, 125/7 pounds of haze, 9/2 pounds of NOx, $9,442.44
recycling one metric ton of steel, could save 11/10 metric tons of iron ore, 630 kilograms of coal, 55 kilograms of limestone, over 1 metric ton of green house gases, a 139,073,760/403 cubic meter lake, keep 4,599/201,500 tons of mercury out of landfills a year, 231/125 metric tons of co2, enough energy to power a CFL for 126/13 years, a 100-watt light bulb for 126/65 years, a tv for 21/130 years, 14π/15 gallons of oil, 12,824π/1,341 kwh of energy, 1,603π/2,500 gallons of gasoline, a 28,000π cubic meter lake, and 56π/15 acres of soil from being polluted, 7,392/5 square meters of natural habitat potential, 105/23 tons of steam, enough energy to power a car to travel 14π miles, 7π/52 cars for a year, 616 kilograms of fossil fuels, 308 kilograms of carbon monoxide, 308/5 kilograms of nitrogen oxide, 2,772/625 metric tons of life, 924/955 metric tons of ethanol
Gold Recycling
gold that's been made out of 100% recycled gold
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of gold readily oxidizes back into gold oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2800 °F to produce molten gold.
- Dross is removed and the dissolved hydrogen is degassed. (Molten gold readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity gold, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 gold alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten gold poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
recycling one ton of gold could save 400,000 tons of waste, 3,200,000 kwh of energy, 806/73 metric tons of coal, 35,464/1,095 metric tons of co2, a 9,600 cubic meter lake, 8,000,000/27 cubic yards of landfill space, and keep 800 pounds of airborne mercury, 3,600,000 tons of cyanide out of landfills, 403,000/5,037 tons of steam, 35,464/3,285 metric tons of fossil fuels, 3,200/39 metric tons of hydrogen, 5,364/5 tons of biomass, 596 tons of carbon, enough energy to power a CFL for 12,400/73 years, a 100-watt light bulb for 2,480/73 years, $384,000.00 in energy
Copper Recycling
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of copper readily oxidizes back into copper oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2800 °F to produce molten copper.
- Dross is removed and the dissolved hydrogen is degassed. (Molten copper readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity gold, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 copper alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten copper poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
energy is saved by 85%
recycling one ton of copper saves 99 tons of waste, 4,752/5 kwh of energy, 531,036/5,725 gallons of oil, 297/1,250 pounds of mercury a year, a 1,782/625 cubic meter lake, 1,188/3,125 tons of coal, 398,277/6,250 gallons of gasoline, 4,752/175 metric tons of toxic lead, 220/3 cubic yards of landfill space, 2,124,144/5,725 acres of soil from being polluted, 1,188/25 metric tons of limestone, 2,376/78,125 tons of fly ash, 198/8,125 metric tons of hydrogen, 594/3,125 tons of sulfur dioxide, 297/15,625 tons of sulfur, 19/10 tons of fossil fuels, enough energy to power a CFL for 9,207/182,500 years, TV for 300/73 years or a computer for 650/219 years, over $825,805.00
Zinc recycling
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of zinc readily oxidizes back into zinc oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2800 °F to produce molten zinc.
- Dross is removed and the dissolved hydrogen is degassed. (Molten zinc readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity gold, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 zinc alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten zinc poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
energy is saved by 60%
recycling one metric ton of zinc could save 3,900 KWh of energy, 52,299/20,000 tons of co2, 87,165/229 gallons of oil, 39/40 pounds of mercury a year, a 117/10 cubic meter lake, 39/25 tons of coal, 261,495/1,832 tons of greenhouse gases, 52,299/200 gallons of gasoline, 780/7 metric tons of toxic lead, 1,625/4 tons of waste, 8,125/27 cubic yards of landfill space, 348,660/229 acres of soil from being polluted, 195 metric tons of limestone, 52,299/40,000 tons of biomass, 5,811/8,000 tons of carbon, 1,248/5 pounds of fly ash, 100 kilograms of hydrogen, 5,239/53,728 tons of steam, 312 pounds of sulfur dioxide, 156 pounds of sulfur, enough energy to power a CFL for 1,209/5,840 years, $468.00
Iron recycling
- Cans are first divided from municipal waste, usually through an eddy current separator, and cut into little, equal pieces to lessen the volume and make it easier for the machines that separate them.
- Pieces are cleaned chemically/mechanically, and blocked to minimise oxidation losses when melted. (The surface of iron readily oxidizes back into iron oxide when exposed to oxygen.).
- Blocks are loaded into the furnace and heated to 2800 °F to produce molten iron.
- Dross is removed and the dissolved hydrogen is degassed. (Molten iron readily disassociates hydrogen from water vapor and hydrocarbon contaminants.) This is typically done with chlorine and nitrogen gas. Hexachloroethane tablets are normally used as the source for chlorine. Ammonium perchlorate can also be used, as it decomposes mainly into chlorine, nitrogen, and oxygen when heated.
- Samples are taken for spectroscopic analysis. Depending on the final product desired, high purity gold, copper, zinc, manganese, silicon, and/or magnesium is added to alter the molten composition to the proper alloy specification. The top 5 iron alloys produced are apparently 6061, 7075, 1100, 6063, and 2024.
- The furnace is tapped, the molten iron poured out, and the process is repeated again for the next batch. Depending on the end product it may be cast into ingots, billets, or rods, formed into large slabs for rolling, atomized into powder, sent to an extruder, or transported in its molten state to manufacturing facilities for further processing.
recycling one ton of iron could save 1 ton of coal saves 2,500 kwh of energy, 1,341/800 tons of co2, a 6,705/7,328 cubic meter container of oil, 10 ounces of mercury a year, a 15/2 cubic meter lake, 167,625/1,832 tons of greenhouse gases, 1,341/8 gallons of gasoline, 500/7 metric tons of toxic lead, enough energy to power a CFL for 155/1,168 years, a city the size of Pittsburg for 219/3,220,000 weeks, a over 21/32 cubic meter lake from being polluted, 2,635,152,838/73 btu's of energy, 3,125/12 tons of waste, 15,625/81 cubic yards of landfill space, 223,500/229 acres of soil from being polluted, 125 metric tons of limestone, 12,069/5,920 tons of air pollution per year, 160 pounds of fly ash, 200 pounds of sulfur dioxide, 1,500 pounds of coke, 468/197 tons of acid rain, 100 pounds of sulfur, 447/1,460 pounds of CFC, $300.00 in energy
recycling one metric ton of iron could save 1 metric ton of coal, 116,800,000/403 kwh of energy, 73/2,015 tons of mercury a year, 44/15 metric tons co2, a 220,752,000/403 cubic meter lake, a 293,679/4,030 cubic meter tank of gasoline, a 848,596,227 cubic meter lake from being polluted, 23,360,000/2,821 metric tons of toxic lead, 7,040/3 square meters of natural habitat potential, 500/69 tons of steam, enough energy to power a CFL for 200/13 years, a 87,600/403 bedroom house for an entire year, 100 watt light bulb for 40/13 years, 44/45 metric tons of fossil fuels, 22/45 metric tons of carbon monoxide, 176/25 metric tons of life, 4,400/207 tons of soda ash, Almost $35,000.00
THE ABCs OF STEEL
Steel and aluminum are both mined from ores, and are made in a similar way. The essential ingredient in steel making is iron ore. Iron ore is plentiful, but we cannot use it as it occurs in nature. Iron is usually combined with oxygen, or with other elements, like carbon and sulfur. We must smelt the iron ore—strip or reduce it of its oxygen—to get to the iron.
It takes a great deal of energy to reduce iron oxides. An oxide is a compound with oxygen and some other element. The reduction takes place in a very hot blast furnace. A chemical reaction takes place in the blast furnace, and the iron is freed from the oxygen. This free iron (called pig iron by steel makers because it forms a pattern that looks like tiny piglets surrounding their mother) is used to make steel.
Steel recycling saves a lot of energy. It is much more energy efficient to use steel scrap to make new steel than to mine the iron ore and then smelt it in a blast furnace at temperatures of 2800 F. It takes about 60 percent less energy to make steel from recycled materials than it does from iron ore. That’s why today’s steel makers always use some steel scrap to make new steel products.
Steel is probably the easiest material to separate from the rest of the solid waste stream. Steel is attracted to magnets, so special magnetic belts can be used to separate steel cans from other recyclables. This is a much more efficient method than the labor-intensive hand-sorting necessary with other recyclables, such as plastics.
Recycling your used steel cans at home is easy, too. All you need to do is rinse the food from the cans. That’s it. Years ago, scrap dealers asked people to remove the paper labels and the tops and bottoms from cans. This is no longer necessary.
If you’re not sure which cans are steel and which are aluminum, use a magnet to separate them. Steel will stick to the magnet; aluminum will not. If you come across a can with a steel body and an aluminum top—called a bimetal can—put the can with the steel recyclables. Steel recyclers can accept all types of steel cans, even those containing aluminum. Aluminum recyclers can only accept 100 percent aluminum cans.
After steel scrap is collected from homes, recycling centers, or waste-to-energy plants, it is shipped to one of the companies that buy old steel—steel mills, iron and steel foundries, scrap dealers, and detinners. Detinners remove the layer of tin from old steel cans. This tin is valuable and can be sold.
Steel can recycling follows almost the same process as aluminum can recycling. Steel cans, along with other steel scrap, are melted in a furnace and then poured into casters that continuously roll and flatten the steel into sheets. Recycled steel cans can be made into new cars, girders for buildings, or new food cans. In the U.S., steel cans and other steel products contain at least 25 percent recycled steel, with some containing nearly 100 percent recycled steel.
Like aluminum, steel can also be recycled again and again. It does not lose any of its strength or quality in the recycling process. It can be a never-ending process that continues to save energy and resources.
References
- ↑ A Guide for Scrap Metal Beginners. Retrieved on 2010-11-12.
Template:Ref
See Also
- Reclaimed metal
- Appliance recycling
- Vehicle recycling
- Aircraft recycling
- Ship breaking
- Scrap
External links
Video
Template:Recycling Template:Metal recycling Template:Transportation recycling Template:E-Cycling Template:Water conservation
